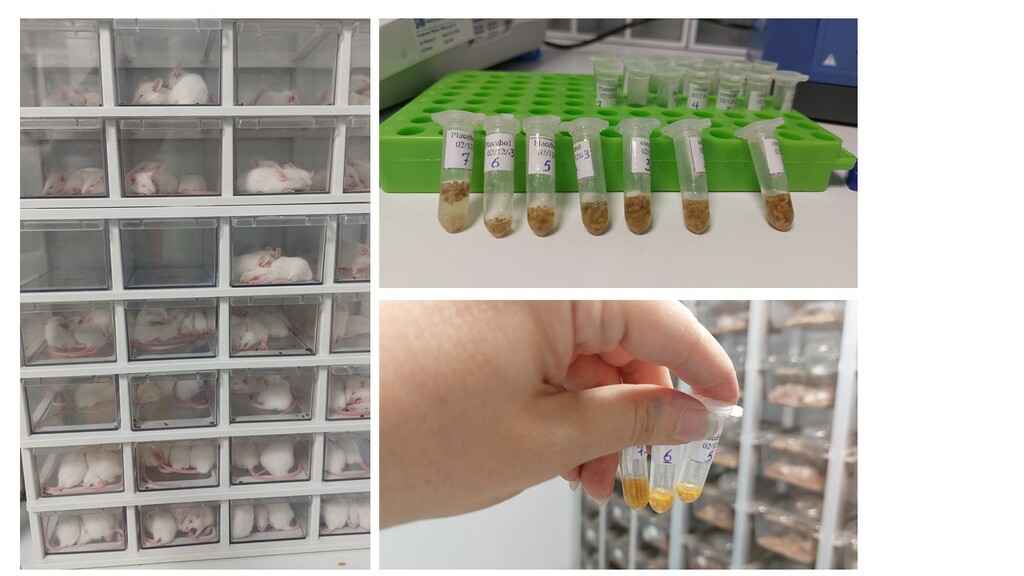
Rahpouyan Fanavar Sadegh has developed ROTASA (bovine-human reassortant vaccine, live, attenuated). live oral bovine-human reassortant vaccine in which human rotavirus strains are reassorted with a bovine rotavirus strain to attenuate their ability to replicate. The most common human G serotypes (G1) are combined with a bovine P serotype to create the strain included in the vaccine.
ROTASA is G1P [8] WI4/9 vaccine that formulated in two viral titrations 106.2 (CCID50) and 107.2 (CCID50) for preclinical study. The non-clinical program included a non-GLP dose-finding study, a viral shedding assessment, and a GLP-compliant toxicity study.
The selection of animal model for the human rotavirus strain has been based previous studies on the susceptibility of Balb/c mice to human strains. these studies revealed that human group A rotavirus was shown to replicate, spread, and induce disease for up to 10 days post-inoculation in neonatal Balb/c mice. Also rotavirus-induced diarrheal disease was shown to be age-dependent in Balb/c mice.
Sobhan Recombinant Protein Company is the manufacturer of the meningococcal vaccine, and the preclinical studies for this vaccine were conducted by the Zistbalin Group. During these preclinical studies, multiple formulations of the meningococcal vaccine were evaluated in laboratory animals to identify the optimal drug dosage and the appropriate ratios of the extracted antigenic polysaccharides. Additionally, toxicological and histopathological assessments were performed.
NanoPlex™ effectively stabilizes vitamins against degradation and enhances their bioavailability under high-acid and high-thermal conditions. It interacts with vitamins and chelates them, preventing degradation during rigorous cooking and processing stages in food and pharmaceutical manufacturing.
At the Zistbalin group, we assess the toxicological effects of NanoPlex on five tissues of Balb/c mice through both single-dose and repeated-dose gavage and intraperitoneal (IP) treatments.
Sodour Ahrar Shargh Knowledge-based Company (SASh Co.) has developed a vaccine adjuvant. An adjuvant is an essential component in certain vaccines that enhances the immune response in individuals receiving the vaccine, effectively improving its overall efficacy.
At Zistbalin Group, we are currently assessing the efficacy and safety of the Ahrar Adjuvant in the Rotavirus Vaccine.
Rahpouyan Fanavar Sadegh has developed a rabies vaccine . At Zistbalin Group we are currently assessing the efficacy and safety of the this vaccine according NIH guideline